CATALYST SCIENTIFIC consists of a team of experienced professionals with strong clinical and regulatory expertise. We provide comprehensive and tailored solutions for targeted pharmaceutical and medical device development. Our team is dedicated to supporting and empowering our clients through personalized attention, innovative technologies, and a commitment to excellence. With our expertise and passion for advancing healthcare, we help you achieve your goals to successfully place your product on the market and improve patient outcomes.
Martin Popovic, Founder and CEO
CATALYST SCIENTIFIC consists of a team of experienced professionals with strong clinical and regulatory expertise. We provide comprehensive and tailored solutions for targeted pharmaceutical and medical device development. Our team is dedicated to supporting and empowering our clients through personalized attention, innovative technologies, and a commitment to excellence. With our expertise and passion for advancing healthcare, we help you achieve your goals to successfully place your product on the market and improve patient outcomes.
Martin Popovic, Founder and CEO

Martin Popovic
Expert for Medical and
Clinical Solutions, and Regulatory Affairs
in the EU and US

Oliver Mauric
Focus on Clinical Research, Medical Devices, and Quality Management

Martin Mayer
Focus on Digital Health,
Regulatory Affairs and
Start-up Strategies

Martin Popovic
Expert for Medical and
Clinical Solutions, and Regulatory Affairs
in the EU and US

Oliver Mauric
Focus on Clinical Research, Medical Devices, and Quality Management

Martin Mayer
Focus on Digital Health,
Regulatory Affairs and
Start-up Strategies
Supporting your entire medical device journey into the US and EU markets
Our team provides expert services to ensure your medical device meets regulatory requirements for successful market launch and remains compliant throughout its lifecycle. We specialize in the EU and US markets, offering:
We take a hands-on approach to ensuring regulatory compliance and success in both EU and US markets by offering tailor-made services that fit your needs and budget. Contact us to learn how we can accompany you at every stage of your medical device journey.

Supporting your entire medical device journey into the US and EU markets
Our team provides expert services to ensure your medical device meets regulatory requirements for successful market launch and remains compliant throughout its lifecycle. We specialize in the EU and US markets, offering:
We take a hands-on approach to ensuring regulatory compliance and success in both EU and US markets by offering tailor-made services that fit your needs and budget. Contact us to learn how we can accompany you at every stage of your medical device journey.

Supporting your entire medical device journey into the US and EU markets
Our team provides expert services to ensure your medical device meets regulatory requirements for successful market launch and remains compliant throughout its lifecycle. We specialize in the EU and US markets, offering:
We take a hands-on approach to ensuring regulatory compliance and success in both EU and US markets by offering tailor-made services that fit your needs and budget. Contact us to learn how we can accompany you at every stage of your medical device journey.
Acting as your reliable in-house PRRC and QRAM
If you are an EU medical device manufacturer or importer, it is important to comply with the Medical Device Regulation (EU MDR) to ensure product safety and effectiveness. We support and strengthen yor team as your in-house PRRC (Person Responsible for Regulatory Compliance) and QRAM (Quality and Regulatory Affairs Manager). Our expert team provides the following services:
PRRC Services (according to MDR 2017/745, Article 15):
QRAM Services:

Acting as your reliable in-house PRRC and QRAM
If you are an EU medical device manufacturer or importer, it is important to comply with the Medical Device Regulation (EU MDR) to ensure product safety and effectiveness. We support and strengthen yor team as your in-house PRRC (Person Responsible for Regulatory Compliance) and QRAM (Quality and Regulatory Affairs Manager). Our expert team provides the following services:
PRRC Services (according to MDR 2017/745, Article 15):
QRAM Services:

Acting as your reliable in-house PRRC and QRAM
If you are an EU medical device manufacturer or importer, it is important to comply with the Medical Device Regulation (EU MDR) to ensure product safety and effectiveness. We support and strengthen yor team as your in-house PRRC (Person Responsible for Regulatory Compliance) and QRAM (Quality and Regulatory Affairs Manager). Our expert team provides the following services:
PRRC Services (according to MDR 2017/745, Article 15):
QRAM Services:
Maximizing market success with comprehensive regulatory due diligence and audit prep services
At CATALYST SCIENTIFIC, we offer comprehensive audit and due diligence services for medical device companies and venture capital investors. Our services involve assessing companies´ regulatory compliance to identify any potential risks that may impact their ability to comply with regulatory requirements. This process includes the review of a product´s development history, clinical evaluation, and risk management activities, followed by remediation planning and ongoing support to meet critical requirements and expectations.
For VCs: Engaging us for regulatory due diligence activities will give you a better understanding of regulatory risks and opportunities, allowing you to make more informed investment decisions.
For manufacturers: We understand the importance of optimal audit preparations and are committed to providing you with the highest level of confidence before audits by Notfied Bodies, EU regulatory authorities or the FDA. Contact us today to learn more about our audit and due diligence services.
Maximizing market success with comprehensive regulatory due diligence and audit prep services
At CATALYST SCIENTIFIC, we offer comprehensive audit and due diligence services for medical device companies and venture capital investors. Our services involve assessing companies´ regulatory compliance to identify any potential risks that may impact their ability to comply with regulatory requirements. This process includes the review of a product´s development history, clinical evaluation, and risk management activities, followed by remediation planning and ongoing support to meet critical requirements and expectations.
For VCs: Engaging us for regulatory due diligence activities will give you a better understanding of regulatory risks and opportunities, allowing you to make more informed investment decisions.
For manufacturers: We understand the importance of optimal audit preparations and are committed to providing you with the highest level of confidence before audits by Notfied Bodies, EU regulatory authorities or the FDA. Contact us today to learn more about our audit and due diligence services.

Maximizing market success with comprehensive regulatory due diligence and audit prep services
At CATALYST SCIENTIFIC, we offer comprehensive audit and due diligence services for medical device companies and venture capital investors. Our services involve assessing companies´ regulatory compliance to identify any potential risks that may impact their ability to comply with regulatory requirements. This process includes the review of a product´s development history, clinical evaluation, and risk management activities, followed by remediation planning and ongoing support to meet critical requirements and expectations.
For VCs: Engaging us for regulatory due diligence activities will give you a better understanding of regulatory risks and opportunities, allowing you to make more informed investment decisions.
For manufacturers: We understand the importance of optimal audit preparations and are committed to providing you with the highest level of confidence before audits by Notfied Bodies, EU regulatory authorities or the FDA. Contact us today to learn more about our audit and due diligence services.
Enabling you to successfully bring your medical device to the market
Our mission is to help start-up companies bring their medical devices to market, by providing strategic, regulatory, and quality management services. We offer efficient and tailored services at every stage of the development process, from concept to commercialization.
Our regulatory experts will help ensure that your device meets all necessary requirements. We can also develop a quality management system tailored to your specific device and company needs.
As part of our support, we can assist in developing a commercialization strategy. This includes identifying potential markets, developing marketing strategies, and building relationships with key stakeholders.
We are passionate about supporting innovation in the medical device industry and helping turn ideas into great products with a positive patient impact. Contact us today to learn more about how we can support your start-up journey.

Enabling you to successfully bring your medical device to the market
Our mission is to help start-up companies bring their medical devices to market, by providing strategic, regulatory, and quality management services. We offer efficient and tailored services at every stage of the development process, from concept to commercialization.
Our regulatory experts will help ensure that your device meets all necessary requirements. We can also develop a quality management system tailored to your specific device and company needs.
As part of our support, we can assist in developing a commercialization strategy. This includes identifying potential markets, developing marketing strategies, and building relationships with key stakeholders.
We are passionate about supporting innovation in the medical device industry and helping turn ideas into great products with a positive patient impact. Contact us today to learn more about how we can support your start-up journey.

Enabling you to successfully bring your medical device to the market
Our mission is to help start-up companies bring their medical devices to market, by providing strategic, regulatory, and quality management services. We offer efficient and tailored services at every stage of the development process, from concept to commercialization.
Our regulatory experts will help ensure that your device meets all necessary requirements. We can also develop a quality management system tailored to your specific device and company needs.
As part of our support, we can assist in developing a commercialization strategy. This includes identifying potential markets, developing marketing strategies, and building relationships with key stakeholders.
We are passionate about supporting innovation in the medical device industry and helping turn ideas into great products with a positive patient impact. Contact us today to learn more about how we can support your start-up journey.
We leverage key opinion leader insights to answer your scientific questions. Well-organized, -moderated and -documented
Advisory Board Meetings are an essential aspect of successful clinical development that is aligned with state-of-the-art scientific knowledge.
We leverage key opinion leader insights to answer your scientific questions. Well-organized, -moderated and -documented
Advisory Board Meetings are an essential aspect of successful clinical development that is aligned with state-of-the-art scientific knowledge.
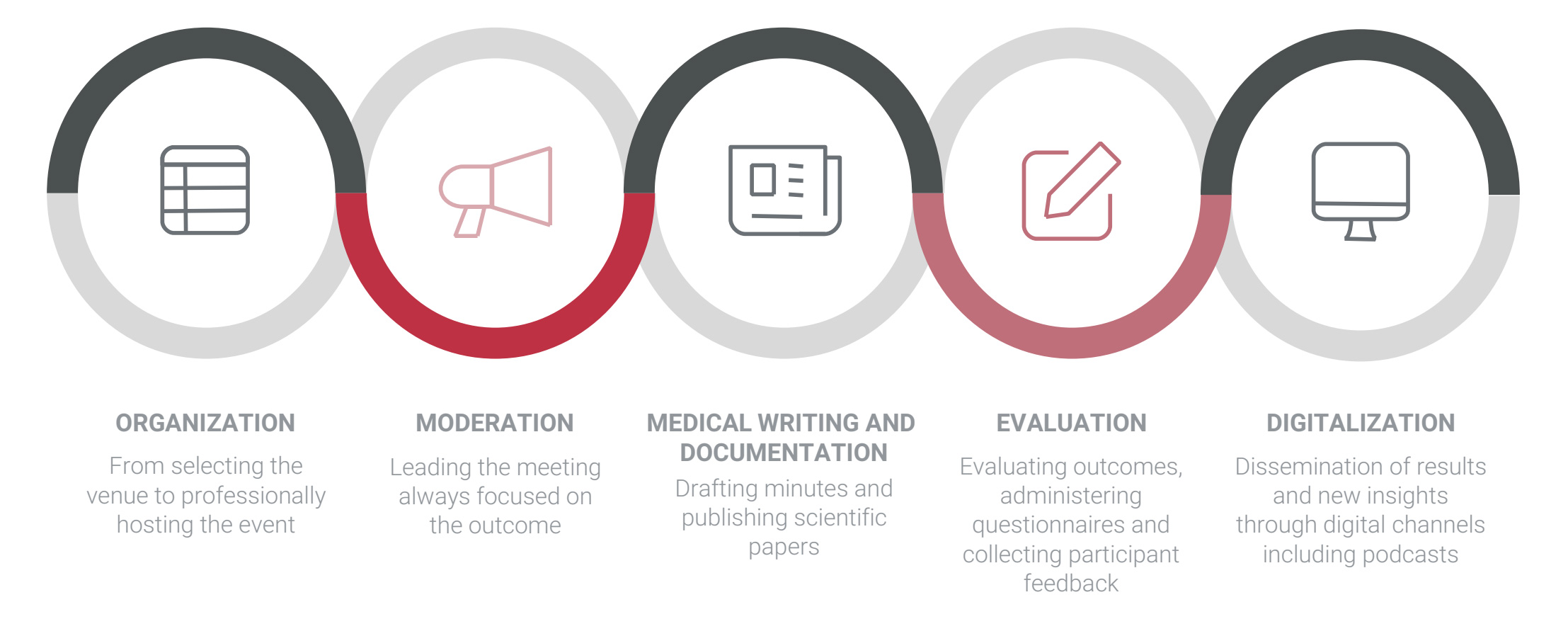
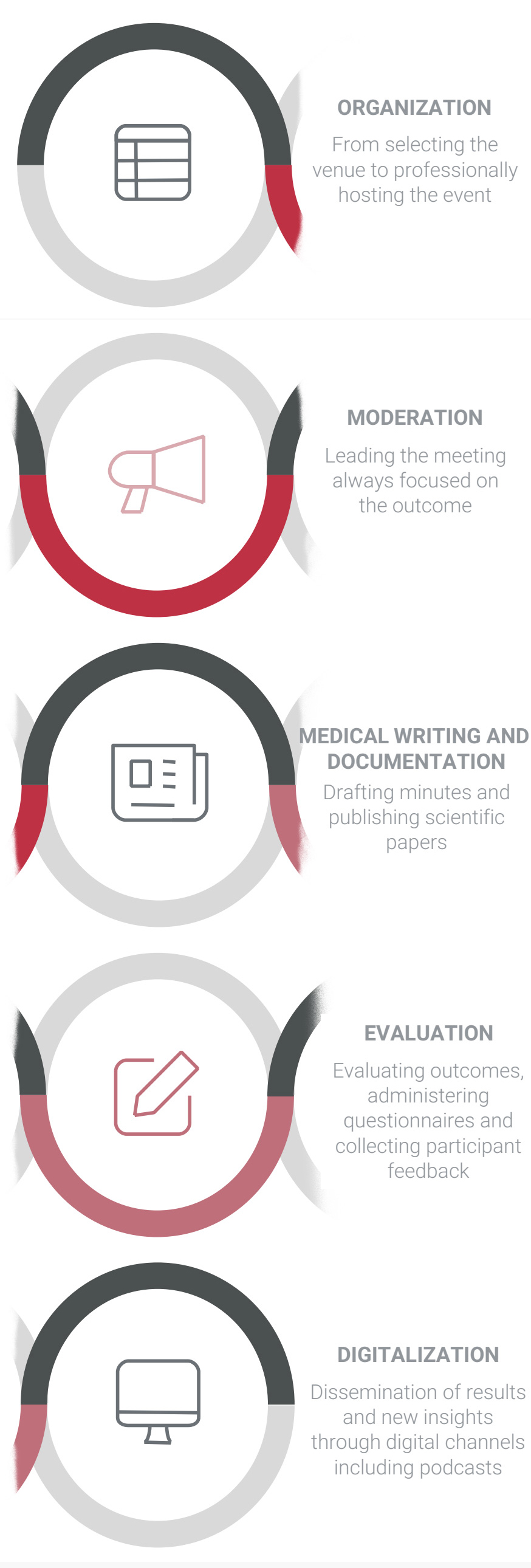
We support your clinical operations team in conducting phase I to IV projects
and we provide expert advice
from planning through execution.
We support your clinical operations team in conducting phase I to IV projects
and we provide expert advice
from planning through execution.
Protocol design
Developing customized protocols that meet regulatory and sponsor requirements
Vendor selection
Partnering with your outsourcing team in finding appropriate and specialized vendors
Country & site feasibility
Assessing regions and sites to identify the right operational partners for your trial
Project management
Supporting your project management teams using best-in-class strategies
Recruitment strategies
Defining the right approach to recruit patients for your study
Regulatory services
Managing submissions and interactions with competent authorities and ethics committees
Protocol design
Developing customized protocols that meet regulatory and sponsor requirements
Vendor selection
Partnering with your outsourcing team in finding appropriate and specialized vendors
Country & site feasibility
Assessing regions and sites to identify the right operational partners for your trial
Project management
Supporting your project management teams using best-in-class strategies
Recruitment strategies
Defining the right approach to recruit patients for your study
Regulatory services
Managing submissions and interactions with competent authorities and ethics committees